Every living cell exists in a delicate balance with its environment. One aspect of this balance is the concentration of solutes in the fluid outside the cell versus inside the cell. Enter the concept of hypotonic solutions. In the realm of biology, understanding such solutions becomes critical to many physiological processes and reactions that keep organisms alive and well. So, what exactly is a hypotonic solution, and why does it matter? Dive in to discover its definition, effects, and some everyday examples.
What is Hypotonic Solution?
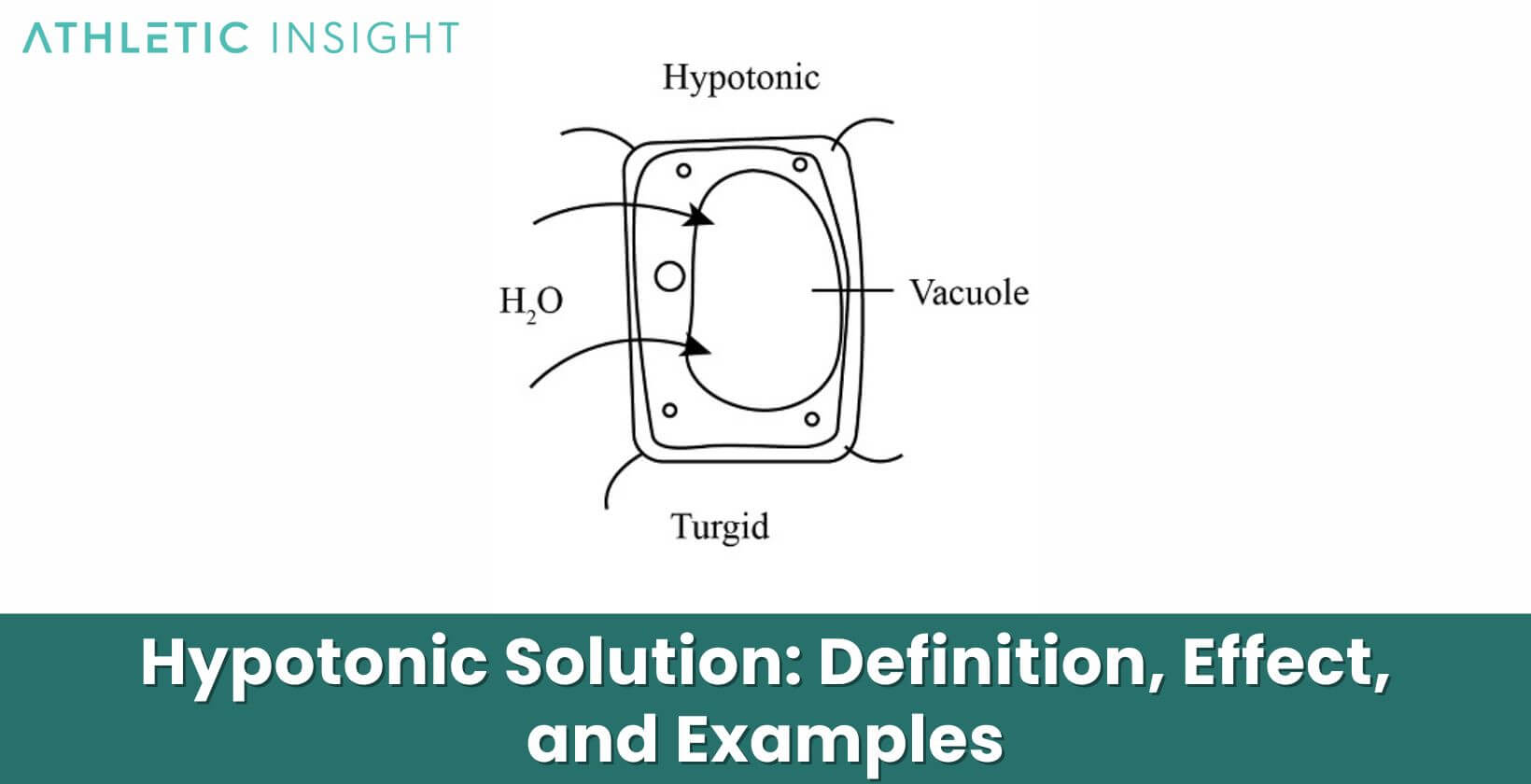
At its core, a hypotonic solution is one where the concentration of solutes (like salts or sugars) is lower outside the cell than inside it. This difference sets the stage for the movement of water. Water molecules, always on the move, tend to flow from areas of low solute concentration to areas of high solute concentration. This natural flow is a process termed osmosis.
When a cell finds itself in a hypotonic solution, it experiences an influx of water. Given that the solution outside the cell has fewer solutes, water moves into the cell, trying to equalize the concentration. This movement can lead to the cell swelling, a pivotal response that has both benefits and potential risks.
How does Hypotonic Solution work?
Cells in a hypotonic solution undergo a fascinating yet straightforward process. Because of the lower concentration of solutes in the solution compared to the cell’s interior, water rushes into the cell. It’s osmosis in action, a passive movement of water molecules seeking equilibrium.
As water continues to flow into the cell, it swells. Imagine a balloon slowly filling with water; that’s what happens to cells. They enlarge as they try to accommodate the incoming water. This process continues until equilibrium is achieved or until the cell’s structural limits are tested.
What is the effect of Hypotonic Solution on the body?
In the grand theater of the human body, hypotonic solutions play a significant role. When cells find themselves in a hypotonic environment, they become the center of attention, attracting water towards themselves. This phenomenon is due to the natural tendency of water to move from an area of low solute concentration (the hypotonic solution) to an area of higher concentration (inside the cell).
While this movement can hydrate cells and aid in nutrient transport, it’s not without consequences. Excessive water influx can cause cells to swell dangerously. In the worst-case scenario, they might even rupture, leading to cell death. This balance between hydration and potential cell damage underscores the importance of understanding and managing the effects of hypotonic solutions on the body.
What is the importance of the Hypotonic Solution?
At its core, the significance of hypotonic solutions can’t be understated in the biological world. Such solutions play a pivotal role in regulating cell volume and maintaining internal cellular environments. The movement of water across cell membranes, driven by these solutions, assists in nutrient transport and waste removal.
When cells find themselves in environments where they need to absorb water, hypotonic solutions come into play. In scenarios where hydration is essential or where nutrient uptake is crucial, these solutions offer a mechanism for cells to intake the necessary water, ensuring their proper function and survival.
How important is Hypotonic Solution in Sports Nutrition?
In the world of sports nutrition, balance is everything. Athletes push their bodies to the limit, leading to significant loss of fluids and essential nutrients. Here, hypotonic solutions play a crucial role. These solutions are crafted to quickly replace fluids and provide immediate energy, aiding in faster recovery and sustained performance.
When athletes sweat, not only is water lost, but essential salts and minerals are also expelled. Drinking a hypotonic solution helps in faster absorption of water and replenishes the lost minerals. As a result, it ensures that athletes remain hydrated, their muscles function optimally, and the chances of cramps or fatigue are minimized.
What are the uses of Hypotonic Solution in the body?
Hypotonic solutions find diverse uses in the human body. Primarily, they serve as a medium for cells to absorb water when they are dehydrated or when there’s a need to balance the internal solute concentration. In simpler terms, these solutions help cells ‘drink’ when they’re thirsty.
Beyond hydration, hypotonic solutions aid in nutrient transport within cells. As water rushes into a cell, it can carry with it essential nutrients that the cell needs to function. Similarly, these solutions help in waste removal. As the cell intakes water, waste products can be diluted and more easily expelled, ensuring cellular health and proper function.
Is a Hypotonic Solution Good for Active Dehydration?
When it comes to dehydration, the immediate instinct might be to think that all fluids can help in rehydration. Is a hypotonic solution good for this purpose? The direct answer is yes. Dehydration implies a loss of both water and essential salts from the body. Hypotonic solutions, by nature, have a lower concentration of solutes compared to body cells.
So, when someone drinks a hypotonic solution during active dehydration, it allows for a rapid intake of water into the body’s cells. This swift absorption helps in quicker rehydration, ensuring cells get the moisture they desperately need. Moreover, it replenishes the lost salts and minerals, ensuring a holistic recovery from dehydration.
What are examples of a Hypotonic Solution?
Understanding the concept of hypotonic solutions becomes clearer when concrete examples are provided. These solutions are abundant in various scientific and real-world scenarios, each serving a unique purpose based on their composition and effect.
Some commonly known examples of hypotonic solutions include plain water (when compared to the inside of our body cells), diluted salt solutions, and solutions used in medical procedures to restore hydration, such as certain IV drips. While plain water might seem like an everyday substance, in the right context, it acts as a hypotonic solution, prompting water to move into cells when consumed.
Is Gatorade a Hypotonic Solution?
Gatorade, a popular sports drink, is frequently consumed by athletes and individuals involved in intense physical activities. When one wonders if Gatorade is a hypotonic solution, the direct answer might surprise some. It’s not. Gatorade is designed to be isotonic. This means it has a solute concentration similar to that of the human body.
The purpose of an isotonic drink like Gatorade is to quickly replace fluids and electrolytes lost during physical exertion. It provides a balance, ensuring that while the body receives the necessary hydration, cells don’t undergo the swelling that they might experience in a purely hypotonic environment.
Is distilled water Hypotonic Solution?
Distilled water is water in its purest form, devoid of minerals, salts, and contaminants. When discussing if distilled water is a hypotonic solution, the answer is unequivocally yes. When compared to the internal environment of human cells, distilled water has a far lower solute concentration.
When consumed, this extremely pure water can rush into cells, driven by the desire to balance solute concentrations. While drinking distilled water in moderate amounts is generally safe, consistently consuming large quantities can potentially disturb the body’s electrolyte balance. Such imbalances might lead to complications, highlighting the importance of understanding the nature of what we ingest.
What are the Benefits of a Hypotonic Solution?
Engaging with hypotonic solutions brings forth an array of benefits, particularly in medical and biological contexts. Firstly, they offer rapid hydration. For cells parched from dehydration, a hypotonic environment ensures water moves into them quickly. This can be crucial in medical emergencies where fast rehydration is paramount.
Secondly, hypotonic solutions aid in nutrient transport. When cells take in water, they also pull in dissolved nutrients. This process ensures cells get the essentials they require for optimal function. Furthermore, in medical scenarios, hypotonic solutions can be used to deliver drugs, ensuring they penetrate cells effectively.
What are the limitations of the Hypotonic Solution?
While hypotonic solutions have their merits, it’s vital to recognize their limitations. The very property that makes them beneficial, their ability to hydrate cells rapidly, can also be a drawback. Excessive hydration can lead cells to swell excessively, risking structural integrity.
Beyond the cellular level, if large quantities of hypotonic solutions are ingested, they might dilute the body’s internal environment. This dilution can lead to a condition called water intoxication or hyponatremia, where sodium levels in the body drop to dangerous levels. Symptoms include nausea, headache, and in severe cases, seizures or coma.
Does a Hypotonic Solution cause swelling?
When discussing the relationship between hypotonic solutions and cellular behavior, one might wonder about the effects on cells. Does a hypotonic solution cause swelling? The straightforward answer is yes. In a hypotonic environment, water moves into the cells, driven by the urge to achieve a balance in solute concentration. This movement results in cells expanding in size.
For many cells, this increased water intake can be beneficial, especially if they’re dehydrated. But there’s a limit. If too much water enters, cells can swell excessively, risking structural integrity. In extreme cases, they might even burst, a phenomenon called lysis. So, while hypotonic solutions can aid in hydration, their effect needs monitoring to prevent potential harm to cells.
What is the difference between Hypotonic and Hypertonic Solutions?
Understanding the distinction between hypotonic and hypertonic solutions is fundamental in biology and medicine. A hypotonic solution, as previously discussed, has a lower concentration of solutes compared to another solution, prompting water to move into the area of higher solute concentration. In the context of cells, this means water rushes into the cell, causing it to swell.
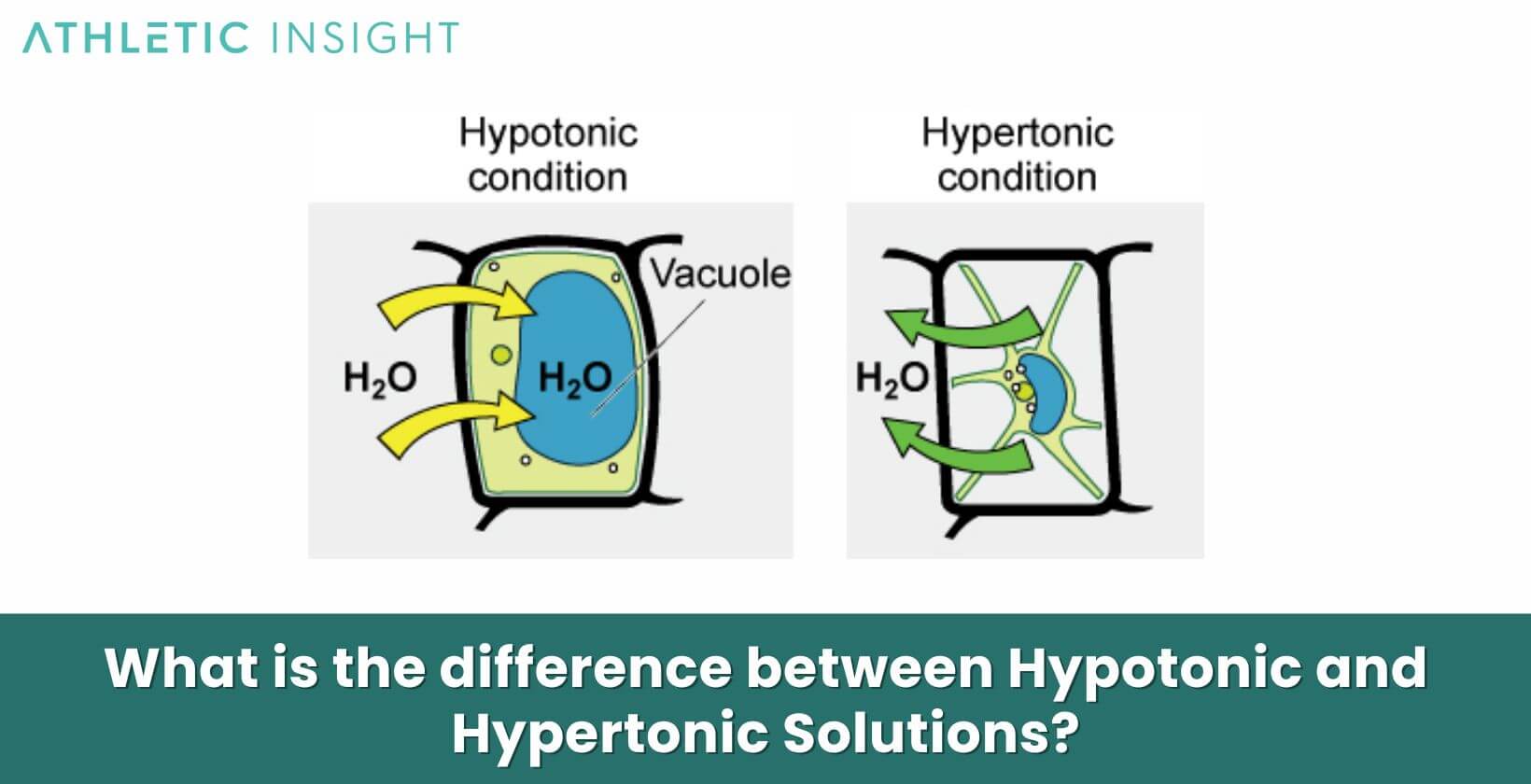
Conversely, a hypertonic solution has a higher solute concentration. When cells are placed in such an environment, they lose water to the surrounding solution, causing them to shrink, a process called crenation. Essentially, while hypotonic solutions make cells swell by drawing water into them, hypertonic solutions cause cells to lose water and shrink.
What is the difference between Hypotonic and Isotonic Solutions?
While both hypotonic and isotonic solutions play roles in regulating cell behavior, they have distinct characteristics. A hypotonic solution, as we know, has a lower concentration of solutes relative to another solution. In relation to cells, this encourages water to move into the cell, potentially causing it to swell.
In contrast, an isotonic solution has a solute concentration equivalent to that of the cell’s internal environment. In this scenario, there’s a balanced movement of water into and out of the cell. This equilibrium means cells maintain their size and shape without swelling or shrinking. It’s a state of balance, where cells remain stable, neither gaining nor losing significant amounts of water.